Podcast: Play in new window
Subscribe: RSS
[display_podcast]
Date: January 19th, 2017
Reference: Keh D et al. Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis: The HYPRESS Randomized clinical Trial. JAMA 2016.
Guest Skeptic: Salim Rezaie is a faculty physician at Greater San Antonio Emergency Physicians (GSEP) in San Antonio, Texas. He is the founder and creator of REBEL EM and REBEL Cast as well as the co-creator and co-founder of the Teaching Institute, a medical education startup company trying to change the world of medical education.
Case: A 66-year-old female comes to the emergency department with fever and cough. She has a past medical history of diabetes mellitus, hypertension, and hyperlipidemia. Initial vital signs show a blood pressure of 154/87 mmHg, heart rate 132 beats per minute, respiratory rate 28 breaths per minute, oxygen saturation of 94% on room air, and an oral temperature of 101.9 degrees Fahrenheit (38.8C). You get two peripheral intravenous lines, place the patient on two litres of oxygen by nasal cannula, and hook them up to the cardiac monitor. You begin with a two litre normal saline bolus, Pan-culture your patient, and give empiric antibiotics for community acquired pneumonia (CAP), as this patient has no risk factors for nosocomial infections. A chest X-ray confirms your suspicion of CAP. Despite aggressive intravenous fluids and antibiotics the patient’s systolic blood pressure starts to trend down into the 140s, then the 120s. You remember reading about a recent study that talked about giving hydrocortisone early in the spectrum of sepsis, before the patient develops septic shock.
Background: We have covered sepsis a number of times on the SGEM.
- SGEM#44: Pause (Etomidate and Rapid Sequence Intubation in Sepsis)
- SGEM#69: Cry Me A River (Early Goal Directed Therapy) ProCESS Trial
- SGEM#90: Hunting High and Low (Best MAP for Sepsis Patients)
- SGEM#92: ARISE Up, ARISE Up (EGDT vs. Usual Care for Sepsis)
- SGEM#113: EGDT – ProMISe(s) ProMISe(s)
One thing we have not covered is the use of steroids in treating sepsis.
The Surviving Sepsis Campaign 2016 was just published updating the 2012 guidelines. The new guidelines continue to give a weak recommendation based on low quality evidence for the use of intravenous hydrocortisone at a dose of 200mg per day in patients with refractory septic shock (i.e. inadequate response to fluid resuscitation and vasopressor therapy).
There have been some changes in the definitions for sepsis and septic shock (Singer et al JAMA 2016. This is important to consider when looking at the study we are going to be reviewing today.
REBEL EM did a summary piece of the Singer et al paper entitled Sepsis 3.0. The overall summary points were:
- Sepsis = life-threatening organ dysfunction caused by a dysregulated host response to infection
- Septic Shock = Need for Vasopressors and Lactate >2 mmol/L
- Severe Sepsis is out
- SIRS is out and qSOFA/SOFA are in
Some of the other terms to know for this episode of the SGEM.
- Systemic Inflammatory Response Syndrome (SIRS) Criteria:
- A temperature over 38C or less than 36C
- A heart rate over 90 beats/min
- A respiratory rate over 20 breaths/min or PCO2 less than 32mmHg
- A WBC count less than 4,000 or over 12,000 or greater 10% immature forms
- Sepsis: At least two of the four SIRS + infection
- Severe Sepsis: Sepsis + hypotension and end organ failure
- Hypoxia, renal failure, hepatic failure, coagulopathy, hypotension or lactate greater than 2mmol/l
- Septic Shock: Severe sepsis and hypotension refractory to fluid treatment or lactate greater than 4mmol/l
We are not going to get into the controversy about the new definitions. There are many #FOAMed resources available that discuss the issue. Rather, this SGEM episode will focus on the idea of using hydrocortisone in patients with severe sepsis.
- FOAMCast: Sepsis – Redefined
- St. Emlyn’s Blog: Holy Smokes! Batman, the SOFA and the Latest Sepsis Definitions
- First10EM: Sepsis 3.0?
- PulmCrit(EMCrit): Top 10 Problems with the new Sepsis Definition
- Intensive Care Medicine Working Knowledge: Sepsis 3 – The Rise of the SOFA
- EMCrit: Sepsis 3.0 with Melv Singer
- EMCrit: Wee – Cliff Deutschman with Additional Thoughts on Sepsis 3.0
- Intensive Care Network: Sepsis is Not a Disease
- PulmCrit(EMCrit): Bad news for Sepsis-3.0: qSOFA Fails Validation
The recommendations for hydrocortisone in refractory septic shock are mostly based on two randomized clinical trials (Annane et al JAMA 2002 and Sprung et al NEJM 2008), but subsequent meta-analyses have had more mixed results (Sligl et al Clin Infect Dis 2009 and Annane et al Cochrane 2015). Shock reversal was consistently improved irrespective of disease severity; however, mortality outcomes were not as consistently improved.
Therefore, it has been hypothesized that early hydrocortisone administration could possibly prevent septic shock by attenuating a patient’s inflammatory response.
Clinical Question: Does the use of hydrocortisone in patients with severe sepsis prevent the development of septic shock?
Reference: Keh D et al. Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis: The HYPRESS Randomized clinical Trial. JAMA 2016.
- Population: Adult patients in intermediate care units or intensive care units from 34 centers in Germany that met all of the following criteria:
- Inclusion: Evidence of infection, had evidence of a systemic response to infection (defined as at least two SIRS criteria) and had evidence of organ dysfunction present for not longer than 48 hours.
- Exclusion: Main exclusion was septic shock (hypotensive despite adequate fluid resuscitation or needing vasopressors for more than four hours). Other exclusions included: younger than 18 years of age, hypersensitivity to hydrocortisone or mannitol, having a history of regularly on glucocorticoids, pregnant, breast feeding, moribund or had a do not resuscitate order.
- Intervention: Hydrocortisone IV bolus of 50mg, followed by a 24 hour continuous infusion of 200mg for 5 days, 100mg on days 6 and 7, 50 mg on days 8 and 9, and 25mg on days 10 and 11.
- Comparison: Placebo (133mg of lyophilized mannitol)
- Outcome:
- Primary: Development of septic shock within 14 days.
- Secondary:
- Time until septic shock or death (whichever came first)
- Mortality in the ICU and hospital
- Vital status at 28, 90 and 180 days
- Duration of stay in the ICU and hospital
- Sequential Organ Failure Assessment (SOFA) score
- Duration of mechanical ventilation
- Renal replacement therapy
- Frequency of delirium
- Adverse Events:
- Development of secondary infections
- Weaning failure
- Muscle weakness
- Gastrointestinal bleeding
- Hyperglycemia (Blood glucose >150 mg/dL)
Author’s Conclusions: “Among adults with severe sepsis not in septic shock, use of hydrocortisone compared with placebo did not reduce the risk of septic shock within 14 days. These finding do not support the use of hydrocortisone in these patients.”
 Quality Checklist for Randomized Clinical Trials:
Quality Checklist for Randomized Clinical Trials:
- The study population included or focused on those in the emergency department. No. These were patients from intermediate units and intensive care units, but these types of patients could be extrapolated to the emergency department, as we often see patients with severe sepsis.
- The patients were adequately randomized. Yes. This was done in a 1:1 fashion using an internet-based computerized randomization tool.
- The randomization process was concealed. Yes. All patients, study personnel, sponsors, medical staff, and nursing staff were blinded regarding the allocation of study medication throughout the entire study period.
- The patients were analyzed in the groups to which they were randomized. No. They did a modified intention to treat analysis.
- The study patients were recruited consecutively (i.e. no selection bias). Unsure. 9,953 patients were screened from Jan 2009 to Aug 2013, but it is unclear from the publication whether screening was consecutively or based on a convenience sample
- The patients in both groups were similar with respect to prognostic factors. Yes. Treatment arms were comparable regarding age, type of admission to the ICU, severity of disease or organ dysfunction, use of etomidate within 72 hours before randomization, initial treatment, and vital signs within 6 hours after diagnosis of severe sepsis.
- All participants (patients, clinicians, outcome assessors) were unaware of group allocation. Yes. Medications were delivered in boxes, containing 17 brown glass vials containing 100mg of lyophylized hydrocortisone or the same amount of lyophilized mannitol (i.e. placebo) that were indistinguishable.
- All groups were treated equally except for the intervention. Unsure. It appears they were treated similarly in terms of antibiotic treatment, volume of crystalloid resuscitation, use of vasopressors. We are not so sure about some other treatments.
- Follow-up was complete (i.e. at least 80% for both groups). Yes. Both groups had a less than 9% loss to follow up rate.
- All patient-important outcomes were considered. Yes. I believe so (i.e. mortality, development of secondary infections, muscle weakness, delirium, and hyperglycemia).
- The treatment effect was large enough and precise enough to be clinically significant. No.
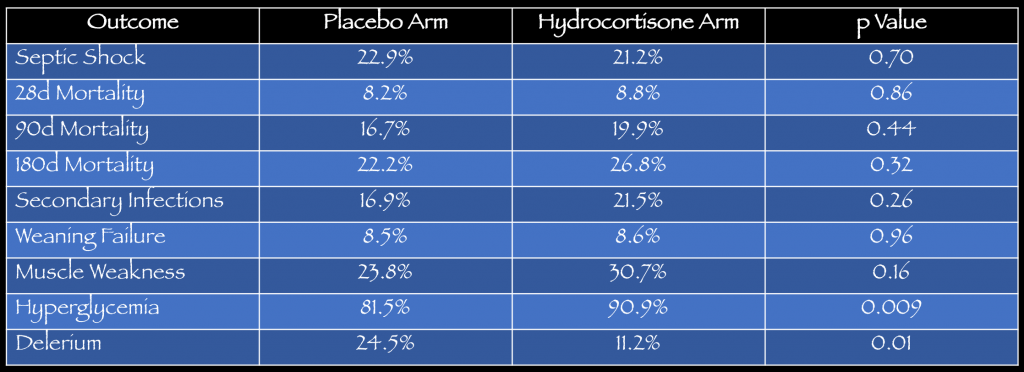
Key Results: There were 9,953 patients with severe sepsis or septic shock screened for inclusion. A total of 380 were randomized to receive hydrocortisone (n=190) or placebo (n=190). The mean age was 65 years with 65% being male. See table below for details on the primary outcome, secondary outcomes and adverse events.
Primary Outcome: No statistical difference in developing septic shock within 14 days.
- Outcomes:
- Primary: No statistical difference in developing septic shock within 14 days. 21% (36/170) hydrocortisone vs. 22.9% (39/170) placebo, p= 0.70.
- Secondary: No statistical differences in mortality at 28, 90 or 180 days. More delirium was noted in the placebo arm (24.5%) vs. hydrocortisone arm (11.2%).
- Adverse Events: No statistical difference in adverse events except more episodes of hyperglycemia with hydrocortisone arm (90.9%) vs. placebo arm (81.5%).
This was a well done, double-blinded, placebo controlled, randomized, multi-centered trial.
1) Statistical Analysis: One of the main issues with this study was the statistical analysis. The study was planned to detect an absolute difference of 15% between the treatment group and placebo group with a significance level of 0.05 and power of 0.8. They assumed 40% of the patients in the placebo group would have septic shock. Then they did a modified Intention-to-treat analysis.
- Over Estimated Prevalence – They over estimate the prevalence of patients in the placebo group that would have septic shock. The assumption was 40% and the observed rate was only 23%. This can happen in research studies but as prevalence goes down the required sample size goes up.
- Effect Size – They designed the study to detect an absolute difference of 15% between the treatment group and the placebo group. It was postulated in part that 15% represented a meaningful difference that could change clinical practice. This would mean a NNT of 7 to prevent one patient from progressing to septic shock. There are many other treatments we provide that have much lower NNTs. Perhaps a 5% difference or NNT of 20 would be enough to change clinical practice?
- This study ended up being underpowered. Check out Josh Farkas’ great post about power on the PulmCrit (EMCrit) blog.
- Modified Intention-to-Treat Analysis: They did not analyse all patients randomized but rather did a modified ITT. This could have introduced bias into the results, which would favour the treatment group.
2) Measurement Bias – Another issue is measurement bias. Progression from severe sepsis to septic shock is not a very precise measure. It is somewhat subjective despite being based on quantitative measures. They did define septic shock as “sepsis-induced hypotension despite adequate volume status for longer than 4 hours (ie, mean arterial pressure <65 mm Hg, systolic arterial pressure <90 mm Hg, or the use of vasopressors to keep mean arterial pressure ≥65 mm Hg or systolic arterial pressure ≥90 mm Hg).” So how much fluid is “adequate” to treat hypotension?
3) Clinical vs. Statistical Significance – Another issue in this study is the issue of clinical vs. statistical significance. Even if the study was properly sized to detect smaller difference (5%) and that was statistically significant it may not be clinically significant.
- Do patients care if they progress from severe sepsis to septic shock? This is a disease-oriented outcome not a patient oriented outcome. A more patient oriented outcome would be survival with good function.
In the end this was an underpowered study that failed to detect a statistical difference in a surrogate marker between hydrocortisone and placebo in patients with severe sepsis. Therefore we cannot reject the null hypothesis.
Comment on authors’ conclusion compared to SGEM Conclusion: We generally agree with the authors’ conclusions with the caveat that it was an underpowered study.
SGEM Bottom Line: The use of hydrocortisone in adult patients with severe sepsis to prevent septic shock cannot be recommended at this time.
Case Resolution: In our patient despite the steadily dropping blood pressure, we choose not to give a dose of hydrocortisone.
Clinical Application: In patients with severe sepsis without septic shock, continue IV fluids, antibiotics and vasopressors. Only consider hydrocortisone to blunt the inflammatory response in patients with refractory septic shock.
What do I tell my patient? Your blood pressure has begun to drop. We are going to try and improve your blood pressure by giving you intravenous fluids and a special drug. This will hopefully work for you and give more time for the antibiotics to help fight the infection. Steroids have been tried in the past but we do not have any good evidence that they work.
Keener Kontest: Last weeks’ winner was…No winner. The question was who discovered human respiratory syncytial virus (RSV)? What other group of viruses did he also discover around the same time? The answer was Robert Chanock who also discovered parainfluenza viruses in late 1950s.
Listen to this weeks’ episode for the keener question. If you know the answer then send an email to TheSGEM@gmail.com with “keener” in the subject line. The first correct answer will receive a cool skeptical prize.
- REBEL EM: The HYPRESS Trial: Early Steroids to Prevent Septic Shock
- The Bottom Line: HYPRESS – Do Steroids Prevent Shock in Patients with Sepsis
- PulmCCM: Corticosteroids for sepsis didn’t prevent septic shock (HYPRESS trial)
- Wiki Journal Club: HYPRESS











You must be logged in to post a comment.