Podcast: Play in new window
Subscribe: RSS
[display_podcast]
Date: March 7th, 2015
Guest Skeptic: Dr. Eric Schneider. Eric is a Community Emergency Medicine Physician in Kansas City, Missouri, who has a drive to bring the most pragmatic, evidence-based and cost-effective care to his patients at an inner city trauma hospital. He’s a father of three, married to a Pathologist, and an avid musician.
Case: 65yo African American male presents to emergency department complaining of swelling of the tongue/lips. It started when he woke at 08:30. He went to Urgent Care and was treated with intramuscular steroids, benadryl and epinephrine. The swelling did not get any better. The patient was referred to emergency department. Swelling started >4 hours ago when you see him. He states no change in the swelling. On examination is vital signs are normal, no stridor, no respiratory distress, no hypoxia but he does have a swollen tongue and slightly slurred speech. He has a history of hypertension and the only medication he takes is the ACE-Inhibitor Lisinopril.
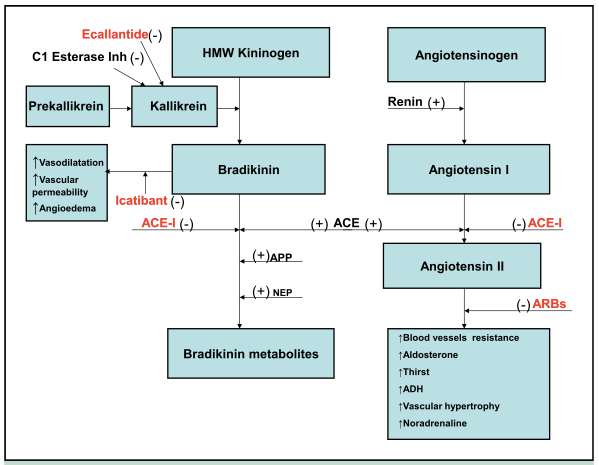
Background: Angiotensin-Converting Enzyme Inhibitors (ACE-Inhibitors), such as Lisinopril, were approved by the FDA in the 1980’s. ACE-I are common medications used for hypertension, congestive heart failure, post myocardial infarction, chronic kidney disease and diabetic nephropathy.
They inhibit the renin-angiotensin-aldosterone system. Angiotensinogen is converted to Angiotensin I by renin. Angiotensin I is then converted to Angiotensin II by angiotensin converting enzyme (ACE) that is mainly produced in the lung.
Angiotensin II has many biological effects including:
- increased blood vessel resistance
- increased aldosterone
- increased thirst
- increased antidiuretic hormone (ADH)
- increased vascular hypertrophy
- increased noradrenaline
Angiotensin converting enzyme also breaks down bradikinin. So if you block ACE with an ACE-I you can get a build up of bradikinin.
ACE-I have side effects like all biologic agents. Because it causes lower blood pressure patients can get hypotension causing people to be weak/dizzy and even cause syncope.
Hyperkalemia is another well-recognized side effect of ACE-I. Urinary potassium excretion is stimulated by aldosterone and ACE-I decrease aldosterone. The estimated incidence of hyperkalemia secondary to ACE-I use is approximately 3%.
Then there is the classic ACE-I cough. This is thought to happen from in 5-35% of patients. This chronic dry could can start after the first dose but interestingly can not show up for weeks, months or even years.
No one knows the exact mechanism by which ACE-I cause cough but it is though to be due to the increased concentration of bradykinin, substance P, prostaglandins and thromboxane. Stopping the ACE-I is the treatment but the cough can persist for months in some patients.
The build up of bradykinin is also though to be the mechanism behind angioedema. The incidence of angioedema is seen in up to 0.7 % of users. It is usually self-limiting and has a very low fatality rate. The angioedema occurs in the mucosal tissue of the tongue, lips, eyelids, GI tract or genitalia that all have rich capillary blood supply.
An interesting fact is that angioedema is up to five times more common in those of African descent.
Angioedema can be divided up into two main types:
- Allergic-histamine-mediated Angioedema:
- Insect bites (bees), foods (peanuts/shellfish), drugs (many)
- Fast onset, pruritus, rash and potential systemic effects
- Responds to anaphylaxis therapy (antihistamines/steroids/epinephrine)
- Non-allergic Angioedema:
- Hereditary angioedema (HAE) and ACE-I angioedema
- Gradual onset, non-pruritic, without rash and effects face, GI tract, genitals
- Responds poorly to anaphylaxis therapy (antihistamines/steroids/epinephrine)
Again, most patients with ACE-I induced angioedema will have mild swelling, no airway obstruction and will resolve within several hours after stopping the drug.
Antihistamines and steroids are often given for the more serious cases of ACE-I angioedema but probably do not have much impact if any because the problem is bradykinin-mediated.
A new drug called icatibant is a bradykinin type 2 receptor antagonist labeled for use with hereditary angioedema. It blocks bradykinin receptors resulting in a rapid reduction of the edema and can prevent the need for intubating patients with significant airway involvement.
Question: Is icatibant of any benefit in ACE-I associated angioedema?
Reference: Bas et al. A randomized trial of Icatibant in ACE-Inhibitor-Induced Angioedema. NEJM January 2015
- Population: Emergency department patients (n=27) between the ages of 18 and 95 who were on ACE-I and exhibited angioedema affecting the upper aerodigestive tract after excluding those with other causes of angioedema,
- Excluded: History of angioedema before ACE-I use, acute urticaria, acute myocardial ischemia, unstable angina, acute heart failure (NYHA class III-IV), or pregnant or lactating.
- Intervention: 30mg icatibant subcutaneously
- Comparison: Intravenous prednisolone 500 mg and clemastine 2 mg
- Outcome:
- Primary: Medium time to complete resolution of edema as evaluated by investigator-assessed and patient –assessed symptom score
- Secondary: Proportion of patients who did not have response to treatment, proportion of patients with complete resolution of edema at 4 hours, time to onset of symptom relief
- Safety: Incidence of and time to rescue intervention, adverse-event reporting, documentation of local (injection-site) reactions, measurement of vital signs and clinical laboratory testing
Authors’ Conclusions: “Among patients with ACE-inhibitor–induced angioedema, the time to complete resolution of edema was significantly shorter with icatibant than with combination therapy with a glucocorticoid and an antihistamine.”
Quality Checklist for Randomized Clinical Trials:
 The study population included or focused on those in the ED. YES
The study population included or focused on those in the ED. YES- The patients were adequately randomized. YES
- The randomization process was concealed. NO, the investigators who were responsible for randomization, study-drug administration, and assessment of injection-site reactions were aware of the study assignments
- The patients were analyzed in the groups to which they were randomized. YES, In the case of 3 patients, the decision to administer treatment was made by the investigator before randomization. These patients were excluded from the results
- The study patients were recruited consecutively (i.e. no selection bias). UNSURE, They did not explicitly state consecutive patients. In addition, three patients were excluded from analysis because physician treatment prior to randomization. Two patients were given icatibant and one patient received standard therapy.
- The patients in both groups were similar with respect to prognostic factors. Mostly, Standard group was older, and there was a mixture of ACE-I’s of unknown significance
- All participants (patients, clinicians, outcome assessors) were unaware of group allocation. NO, the investigators who were responsible for randomization, study-drug administration, and assessment of injection-site reactions were aware of the study assignments
- All groups were treated equally except for the intervention. YES
- Follow-up was complete (i.e. at least 80% for both groups). NO – 4/14 (29%) standard therapy lost to 14 day follow-up.
- All patient-important outcomes were considered. NO, what about need for airway management, admission to hospital, cost, etc?
- The treatment effect was large enough and precise enough to be clinically significant. YES powered at 90% to meet expected difference, P<0.05.
Key Results:
- Primary Outcome: Median time to complete resolution was 8hrs (3-16 range) icatibant vs. 27.1 (20.3-48) for standard care
- Secondary Outcome:
- Complete resolution of edema at 4hr after treatment was 5/13 (38%) for icatibant vs. 0/14 (0%) standard care
- Median time to onset of symptom relief (according to a composite investigator-assessed symptom score) was 2hr (1-8.1) for icatibant vs. 11.7 (8-18) for usual care

- Safety:
- Three patients in standard-therapy group required rescue therapy (icatibant and prednisolone)
- One of these patients needed a tracheotomy
- There were some minor injection site reactions of redness, swelling, pain and itching (see table)

This was a very small study of only 27 patients using icatibant for the off-label use of ACE-I angioedema. Here are five things to consider when evaluating this study:
1) Consecutive Enrolment: We were not sure whether there was consecutive enrolment in this study. This is important to minimize selection bias.
They report three patients had treatment initiated before randomization. Were there other patients that were either too sick or not sick enough who were excluded? They did remove these patients from the efficacy data set and did a per-protocol analysis.
Another point to make about enrollment was all of the patients were Caucasian. ACE-I angioedema is five times more common in patients of African descent. Will they respond differently to icatibant?
2) Blinding: The study was partially un-blinded. Specifically the study drug administrators and the assessors of injection site reactions knew which group patients were assigned. Why were they not blinded? This could introduce some bias when determining whether the active drug or saline subcutaneous injection cause a local reaction. However, the patients and the investigators who assess efficacy outcomes were blinded to treatment groups.
3) Patient Oriented Outcomes: The time to complete resolution 8hrs vs. 27hrs (19hr difference). Median time to onset of symptom relief was 2hr vs. 12hrs.
What about intubation or death? This was a much too small study, which they acknowledge, to assess these rare but very important patient oriented safety outcomes.
How about the need for admission? This might be a very patient oriented outcome. What about cost? These drugs cost $5,000-$10,000. Talk about a very expensive drug to use when most cases are mild, self-limiting, resolve in hours and rarely result in airway compromise.
There is a very big risk of indication creep and that everyone with some facial swelling will be treated with this drug rather than those rare patients heading towards airway obstruction.
4) Usual Care: The standard care was IV steroid (prednisolone) and antihistamine (clementine). I wish they had included epinephrine. This is because if someone is crashing or I’m not sure if it is ACE-I vs. anaphylaxis I would give epinephrine a try. I might also consider fresh frozen plasma.
5) Funded by Shire: Shire did not have a role in the study design. However, Shire did review and provide comments on the manuscript before submission for publication. However, just because a study is pharma funded does not mean the results are wrong but it raises my skeptical radar.
Comment on Authors’ Conclusion Compared to SGEM Conclusion: Icatibant had significantly shorter time to symptom improvement and resolution of edema. What I don’t know is if this therapy will prevent intubation or surgical airways. I can’t generalize to a population of African Descent, who is disproportionately affected. And I can’t necessarily generalize to standard care that may include epinephrine or fresh frozen plasma.
SGEM Bottom Line: Icatibant is an expensive drug that appears to work well for the off-label use of ACE-I induced angioedema but should be reserved for those rare cases of impending airway compromise.
Case Resolution: This gentleman’s swelling had not gone down over a course of a few hours with Epinephrine, Steroids and Antihistamines. He had no respiratory distress. He was not intubated as he had been stable over hours, but given severity of tongue swelling, he was admitted for observation with a trach kit at bedside, with ENT and Anesthesiology aware of him. He had no deterioration, did not require intubation, and went home the following day, knowing not to take any more ACE-inhibitors.
Clinically Application: I am not able to use this clinically because it is not available in places I work.
What Do I Tell My Patient? You have swelling of your tongue, which is likely caused by your medication, Lisinopril. This can occur any time after starting the medicine. There is a medication that may decrease the swelling of your tongue, but I don’t know if it will improve your breathing such that you won’t need your airway protected. Harms of the medicine may include pain, swelling, itching and redness at the injection site. One dose of this medicine costs thousands of dollars.
Keener Kontest: Last weeks’ winner was Dr. Hector Singson from Freeport, Bahamas. He knew that James Wright of the Wright Staining method coined the platelets. This is a back-to-back win for Hector so he will be getting a double strength cool skeptical prize.
Listen to the podcast to hear this weeks keener question. If you know the answer send me email to TheSGEM@gmail.com with “keener” in the subject line. The first person with the correct answer will receive a cool skeptical prize.













You must be logged in to post a comment.